Computational Physical Organic Chemistry and Biochemistry Research

Specific projects are described below.
Mechanisms of Azide Chemistry
Amide moieties, particularly lactams, are common intermediates in natural product synthesis and thus, a natural target of synthetic research. A common method used for the synthesis of amides is the Schmidt reaction between hydrazoic acid and ketones. The utility of the Schmidt reaction was long thought to be limited to the use of hydrazoic acid, as early work showed that no product or very little product was obtained when alkyl azides were employed. It wasn't until the early 1990's that an intramolecular version of this reaction was found to give high yields with the use of an appropriate Lewis acid.
The exact mechanism of the classic Schmidt reaction and the reason why alkyl azides do not work in this reaction are still unknown. The answers to these questions could provide suggestions on how to further improve the usefulness of these reactions and lead to new modifications that allow a wider variety of substrates to be employed. We are using ab initio calculations to determine the mechanisms of these various reactions and to provide an explanation for the difference in reactivities of hydrazoic acid, alkyl azides and intramolecular reactions.

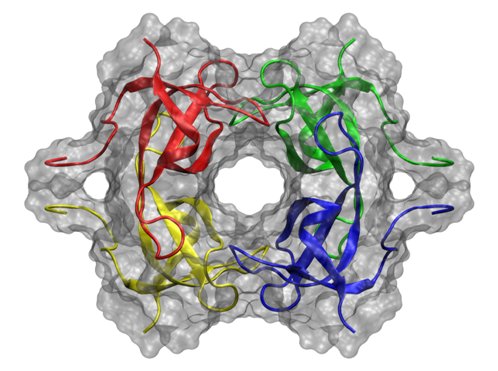
R67 DHFR Binding Cooperativity
R67 dihydrofolate reductase (R67 DHFR) is a plasmid-encoded enzyme that catalyzes the same reaction as DHFR. The protein is a homotetramer and forms a ring with the active site in the center. The reduction of dihydrofolate requires the presence of a co-factor (NADPH). Due to its symmetry, the protein uses identical binding sites to bind dihydrofolate (DHF) and NADPH. To ensure that the productive complex (DHF/NADPH) is formed instead of inhibitory complexes (DHF/DHF or NADPH/NADPH), the protein employs binding cooperativity. However, how the enzyme is able to achieve this cooperativity is unclear. Molecular dynamics simulations are being performed to determine how the enzyme achieves this cooperativity. Comparison of these simulations should provide useful insights into the unique binding properties of this enzyme.
PNA Binding to DNA
Several modifications to the backbone of peptide nucleic acid (PNA) show increased binding to DNA or RNA and show a significant difference in binding affinity between complementary strands and strands containing a mismatch. Thus, PNAs may be potentially useful in diagnostic testing, sensor arrays and antisense applications. The goal of this project is to understand how these modifications cause this change in behavior and to use this knowledge to propose new and improved alterations. Successful modifications not only decrease the flexibility of the PNA, which reduces entropy loss upon duplex formation, but can also alter the conformational profile of the PNA backbone, such that the dihedral angles necessary for duplex formation are more energetically accessible. We plan to use molecular dynamics simulations of both single stranded molecules and double stranded complexes to test this theory. In particular, this project will focus on the replacement of the glycine unit by cis or trans cyclopentane or cis or trans cyclohexane, or the addition of a methyl group at the gamma carbon. This series of compounds spans a wide range of behaviors and should provide significant insight into the relationship between backbone alterations and the resulting properties of the PNA. In addition, we will investigate whether the location of the modification and the specific base attached at the modification have any effect on binding energies.
2-APB Inhibition of SOCC's
2-Aminoethoxydiphenyl borate (2-APB) inhibits calcium from entering store-operated calcium entry channels (SOCC). Other channel types such as voltage-gated channels and receptor operated channels appear to be unaffected by 2-APB. This makes 2-APB a good starting point for locating new or better inhibitors of calcium signaling. Thus, the properties of 2-APB are being studied by ab initio methods. Our initial calculations on 2-APB found that the B3LYP level of theory is particularly poor at representing boron-nitrogen dative bonds and that the MP2 level of theory is the more appropriate choice. We are currently investigating the effect of substituents on the boron-nitrogen dative bond to see if the energy of this bond correlates with SOCC inhibition.
Other Projects
Several small collaborative projects with experimental chemists are also underway.