Hunting Hydnora, or, Finding Furtive Flowers
Few plant families have members as weird as the Hydnoraceae-the botanical equivalent of underground agents. All are holoparasites, that is, plants without chlorophyll that are totally dependent upon their hosts for food and water. Except for flowering, their whole life is spent below the soil surface. The plant body consists of little more than a root and buds, ample equipment for this subterranean creature. No leaves or leaf-like structures are known, a feature unique among angiosperms. Because parasitism and the often associated morphological reduction are usually considered advanced features, the Hydnoraceae could be specialized. However, modern molecular phylogenetic studies show they are among the most primitive of all angiosperms--placed with such "palaeoherbs" as water lilies. These unusual dicots have a pollen grain described as monocolpate, which means that the aperture is long and grooved (See figure).
A small family, the Hydnoraceae comprises only two genera. Prosopanche, with two species, occurs in Central and South America. Hydnora is African and is one of a guild of plants that reaches its northern limit in the southwest corner of Saudi Arabia. The African-South American connection strongly suggests an ancient Gondwanaland distribution. Perhaps because they spend almost their entire lives below ground and perhaps because they are African, Hydnora species have been poorly studied. Best known is Hydnora africana, which parasitizes Euphorbia species in South Africa. The most widely distributed is Hydnora johannis (also known as H. abyssinca) which is found in a broad band across Africa from the Ethiopian Highlands, Nile Valley of Sudan, Uganda, through Zaire and into Namibia (see slide 1). It is a parasite of Acacia trees. One species occurs in Madagascar, H. esculenta, but it has not been seen for many years and, like too many of that island's denizens, may be extirpated.
Certainly the most remarkable of these extraordinary plants is H. triceps. It was first described by Drège from near Okiep in Namaqualand in the Northwestern Cape region of South Africa. Our colleague, Professor Johann Visser, who spent the last part of his life studying parasitic plants in southern Africa, rediscovered H. triceps in 1988--more than 150 years after Drège and more than a century since anyone at all had seen it! In a survey of herbaria, Visser found that the species had been collected less than ten times. Tragically, Visser died shortly after his discovery.
Armed with Visser's field notes and encouraged by our previous work with him, we set out for Namaqualand in September 1999 determined to be the second and third persons to see the plant in a century. September is the ideal time to visit Namaqualand for the incredible display of wild flowers, especially the fabled stone plants. However, it was exceptionally cold and wet when we came to the general area where Visser had seen the plant.
The dominant vegetation in the sandy soil of these low hills is shrubby species of Euphorbia (see colored slide 2). Hydnora triceps has only been found parasitizing Euphorbia dregeana. We located the host. Scattered around the base of the shrubs were telltale fragments of Hydnora, apparently dug up by small mammals. We had found our prey but had yet to see flowers.
What is so amazing about H. triceps is that it flowers underground! Like a pile-driver in reverse, the perianth lobes are united to form a piston that can crack the soil crust without sand entering the flower. Despite its soil borne existence, H. triceps probably depends on flying insects for pollination. The only link between the nether world and pollinators is a distinctive vent-like opening, formed by the perianth lobes which are pink when fresh (see colored slide 3).
We had several objectives in our search: pollinators, fruits, hosts, and relationship of this species to others in the genus and family. Visser had made some observations on floral visitors. He found dermistid beetles (also known to visit H. africana), blowflies (carrion feeding flies of the genus Lucilia), and fleshflies (species of Sarcophaga) in the flowers, not surprising considering their odor. (The strongly fetid odor from our specimens lingered in the car!) We also found beetles and fleshflies and, in addition, ants. Ants are seldom implicated in pollination, but the ants we collected (but have not yet identified) were covered with pollen, especially their mouth parts (see scanning electron micrographs) suggesting they were harvesting pollen. Additional work is needed to determine if these floral visitors are actually pollinators.
In addition to its reluctant entrance into the upper world, H. triceps is also distinguished from other species in the genus by what appears to be a "chamber flower." This is suggested by the androecium (the male parts of the flower) fused into a complex ring through which the pollinator must pass to enter the chamber below (see colored slide 4). Prosopanche americana has many similarities (see colored slide 5, a group of P. americana flowers with perianth lobes at top. Younger flowers on right). Professor A. Cocucci has shown that P. americana heats up at dusk to release a compound that attracts pollinators. Weevils and nitulids enter the flower while the stigmatic surface is receptive and spend the night. At nightfall, a remarkable change takes place in the flower. Stigma receptivity is turned off. "Maleness" is turned on and the pollen is shed. Twenty four hours later, the insects leave the Prosopanche flower dusted with pollen. Perhaps H. triceps has a similar strategy. The presence of chambers in the Namaqualand parasite is in contrast to the two other African species which do not have a constrictive androecium through which insects must pass to receive the stigmatic surface.
No fruits of H. triceps have ever been described. We did not find any, no doubt because we were too early. However, we asked some local shepherds about the fruit. They told us that they collected the fruits and roasted them over coals to be consumed like potatoes apparently unlike the fruits of H. johannis and H. africana which have sweet, delicious fruits eaten raw.
The only host for H. triceps that we found was, appropriately, Drege's spurge-- Euphorbia dregeana. Euphorbia mauritanica, also a large shrub, was abundant but was never a host. Euphorbia mauritanica has a broad distribution throughout South Africa while E. dregeana is restricted to the area near the South Africa-Namibia border (see map). Hydnora triceps is only known from a very restricted area south of the Orange River but should be searched for in Namibia.
A better understanding of pollinators, fruits, and host specificity will require the proverbial further research. Molecular studies are underway to elucidate the relationship of H. triceps with other members of the family.
One of the strangest plants in the world might seem secure away off in its underground habitat in the semidesert of Namaqualand. However, even a brief visit to the site quickly shatters this idea. Diamond mining has devastated large regions. Less obvious threats include the poisoning of predators of livestock by ranchers in the area. This could impact small mammals that feed on the fruits and scatter the seeds. "Out of sight, out of mind" is not a good policy for ensuring the future of one of our planet's most curious plants. Its restricted host and geographic ranges make it especially susceptible to extirpation. And because we know nothing about growing it, the only means of protection is through habitat preservation.




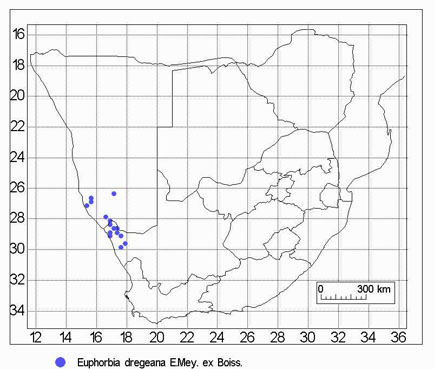
Distribution of the host of Hydnora triceps.
Adapted from: Plant Talk 21: 38-39

This is an ant, as yet unidentified, collected from within the flower of Hydnora triceps (Musselman and Vorster, 99206, 11 September 1999). Note the abundant pollen.

Detail of ant mouth parts, covered in Hydnora triceps pollen.

Monocolpate pollen of Hydnora triceps

Pollen of Hydnora triceps